An azine is the product of condensation of two ketone/aldehyde molecules (carbonyls) and hydrazine. In this post we will be preparing one with vanillin, because its the most accessible aldehyde, and because it exhibits solid-state fluorescence, which is pretty neat.
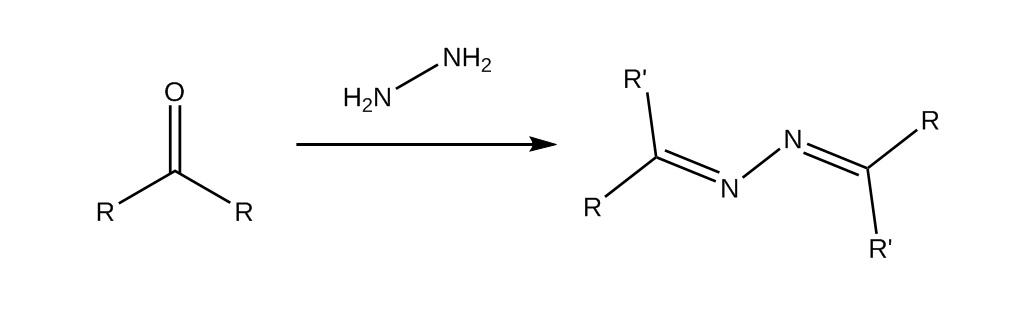
The procedure is taken from a paper and adapted to 0.01 mol scale.
0.02 mol of vanillin (3.04 grams) were placed in a erlenmeyer flask with 50 ml of technical grade ethanol and stirred until dissolved. To the resulting vanillin solution a few drops of Hydrazine Hydrate were added and stirring was continued for the 30 minutes.
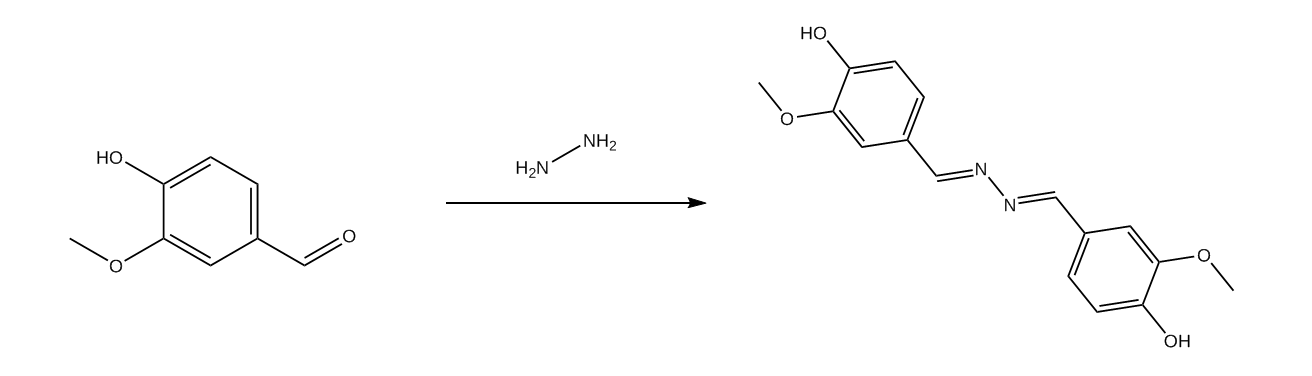
The vanillin azine was precipitated via addition of cold water, and filtered on a sintered glass funnel.

After drying, the azine was placed in an erlenmeyer flask and an excess of 15% hydrochloric acid was added to it, the resulting suspension was stirred and heated for 30 minutes. The formation of a hydrochloride salt caused the azine to change colour from a cream-yellow to bright orange. At this step the product exhibits orange fluorescence under UV unlike the freebase azine.

The resulting orange product was gravity filtered and a yellow solid was obtained. The yellow solid was ground in a mortar and pestle and bits of filter paper stuck to it were removed with a metal sieve. Resulting vanillin azine was placed into a vial.

Further Reading and Applications
Besides having a fluorescent hydrochloride salt, vanillin-azine also can exhibit weak chemiluminescence in a DMSO-NaClO bleach system. I was not able to achieve it, however adding an oxidiser to a solution of vanillin-azine in DMSO has yielded a very fluorescent solution.
Vanillin-azine can also form a red complex with zinc and an orange complex with lead in a basic solution.