This synthesis has started out as an attempt at making Ethylenediammonium Tetrachlorocobaltate (enH2)CoCl4, but due to wrong stoichiometry, another compound with cobalt in the oxidation state of (III) instead of (II) has formed.
While it was carried out a few months ago, only recently have I been able to find a similar compound in literature, more or less confirming it’s identity.
Synthesis
A solution of Cobalt (II) Chloride hexahydrate has been prepared by adding 3 grams of dilute (15%) hydrochloric acid solution to a suspension of 1.51 grams (0.012 mol) of Cobalt (II) Carbonate and boiling the solution to near dryness.
Another solution, of ethylenediammonium dihydrochloride was prepared from an aqueous solution of 1.44 grams of ethylenediamine (0.024 mol) and 7 grams of 15% hydrochloric acid (0.028 mol).
The solutions were combined, and boiled down until nearly dryness, during the evaporation a colour change from red to purple to a deep blue could be observed, indicating the formation of the [CoCl4]2- ion, and a dark blue solid has formed.

However, at this point everything has started to go awry, on cooling the blue colour of the precipitate has reversed to the purple colour characteristic to Co (II), so the solution was set to heat again.

A thick solution of the compound has formed, and acetone was added to wash it away from the beaker and set to vacuum filter, at which point the solid has changed to a green colour.

The green product was filtered off, and washed with extra acetone. In a bout of confusion, in hopes of trying to restore the blue colour, the dry product was ground into a blueish-green powder.
On an attempt to redissolve the product in water, it hydrolysed into an orange compound, characteristic for Co (III)
What went wrong and what is the product?
The product was set aside for a month or so, marked as “mystery cobalt complex”, until a literature review has revealed the existence of a very similar looking product.
In (Coordination Chemistry, Basics and Current Trends, Weber 2023) I have noticed a very familiar looking complex in a sealed ampoule.
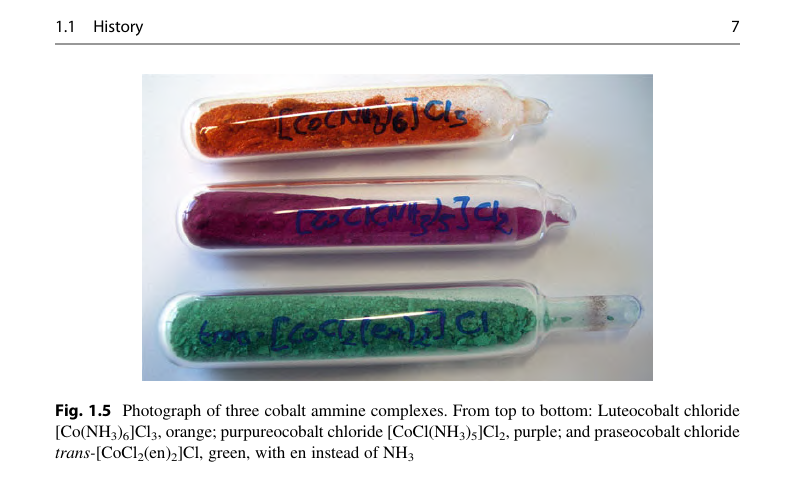
The mystery has been solved! The product is trans-Dichlorobis(ethylenediamine)cobalt(III) chloride, or Praseocobalt (III) Chloride. The usual preparation of which entails reacting ethylenediamine with cobalt (II) chloride in a hydrochloric acid solution and bubbling air through it.
What went wrong is the wrong stoichiometry in the start, where 2 times the needed molar amount of ethylenediamine and not enough hydrochloric acid has been used.
Conclusions
While the original synthesis plan has failed, we still have ended up with an aesthetically pleasing end product, and in pretty high amounts. The synthesis of the ethylenediammonium tetrachlocobaltate will be reattempted at some point to see whether if it exists at all.