This post is partially inspired by one of my first chemistry experiments, an attempt at making a Hydrazine Tetrachlorocuprate from a 1:1 ratio of Copper Chloride and Hydrazine Dihydrochloride that failed because of the complicated Redox Behaviour of Hydrazine - Copper systems.
This preparation is based on a 1979 paper by Brown et al., which details the synthesis of the trichlorocuprate and various other salts. I have picked the trichlorocuprate because of a reported bright green colour and an interesting mixed-ligand coordination sphere in its structure.
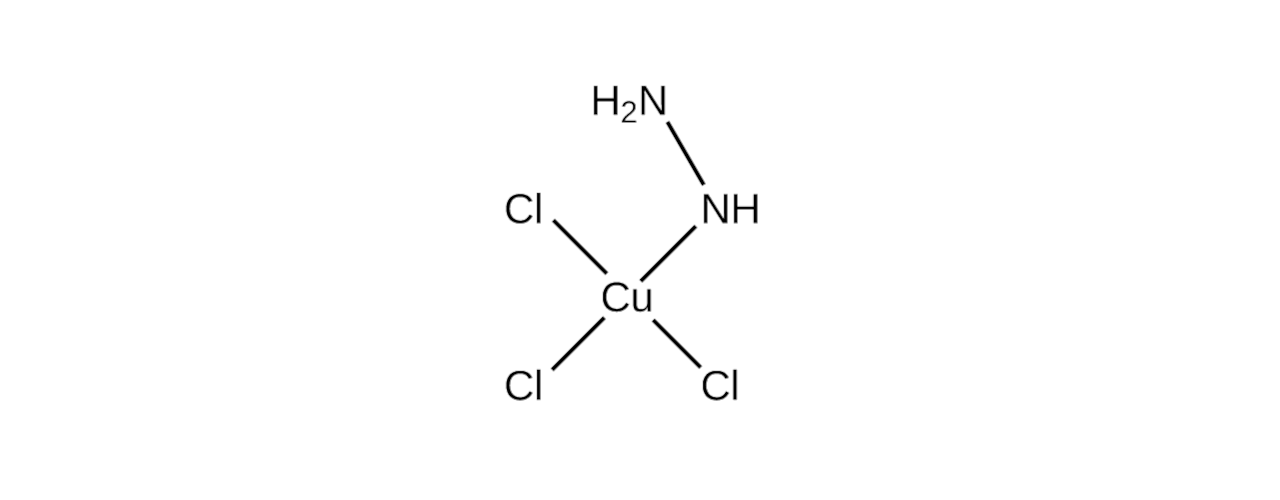
Synthesis
Attempt 1 (successful, with some mistakes)
On the first attempt a solution of 1.87 grams of Copper (II) Chloride and 7 ml of 15% hydrochloric acid in 10 ml of water was prepared. With strong stirring and heating, 1.15 grams of solid Hydrazinium Dihydrochloride were added, and a bright green precipitate reminiscent of Nickel Chloride Hexahydrate has appeared. The solution was hot filtered, and an amount of bright green-blue impure solid remained on the filter paper, with the dark green solution set aside.

Initially this attempt was deemed as a failure, as the bright green precipitate had an amount of impurities, like undissolved crystals of hydrazinium dihydrochloride and blue crystals of unreacted Copper (II) Chloride, but on standing and cooling the dark green solution has formed needle-shaped crystals, which were filtered off and dried.
Attempt 2 (failure)
A second attempt was conducted, with the hydrazinium dihydrochloride finely divided in a mortar and pestle before the addition, with a smaller amount of copper chloride and water used. The solution ended up overheating, however this might’ve not influenced the end product. What doomed this attempt is lack of attention, as by letting it evaporate for too long some amount of impurity crystallized alongside it, and after a few attempts at recrystallization the complex has decomposed.

Results
The final result was a small amount of a green solid, notable for forming an odd white layer smelling strongly of hydrazine (!) on the display vial it was stored in.
