In the year of 1783, Juan Jose and Fausto Elhayar were able to prepare from tungsten-containing starting material a salt that they described as bitter, spicy and yellow (que tenian un sabor picante y amargo)1 2, finally separating tungsten as a separate element after it’s discovery in 1781 and starting the field of polyoxometalate chemistry in the process. After a while of mucking about with silicates and phosphates and molybdenum, modern polyoxometalate chemistry was started for real this time by Joseph Keggin3, with him finally discovering the appropriately named Keggin structure (alpha-Keggin to be precise) via X-ray diffraction of the aforementioned tungstophosphoric acid, finally making inorganic chemists care about more complex shapes other than the usual octahedrons and tetrahedrons.
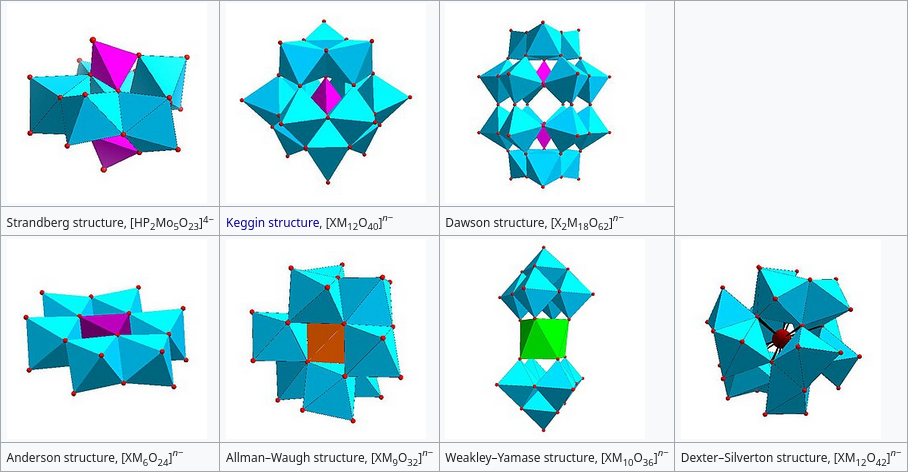
Polyoxometalates are a fascinating and somewhat niche (outside of research) field of inorganic chemistry study. Continuing on from my previous tangent, group 5 and 6 metals can due to their large atomic radii, high oxidation states and high charge also assemble into large complex structures in a way that is somewhat reminiscent of boron chemistry. Here’s the phosphotungstate ion provided as an example.
The chemistry of POMs is relevant in all the same ways as inorganic chemistry research is in general, with the most prominent role given to their usage in catalysis. By incorporating catalytically active metals into the structure of your POM, you are provided an ability to adjust sterics and other properties to your liking akin to modifying an organic ligand in a “classic” coordination complex.
Another application of POMs is subjecting auto structure generators on supplier websites to Insane Psychological Torture and causing the output to look like a pile of spilled metal-oxygen spaghetti:
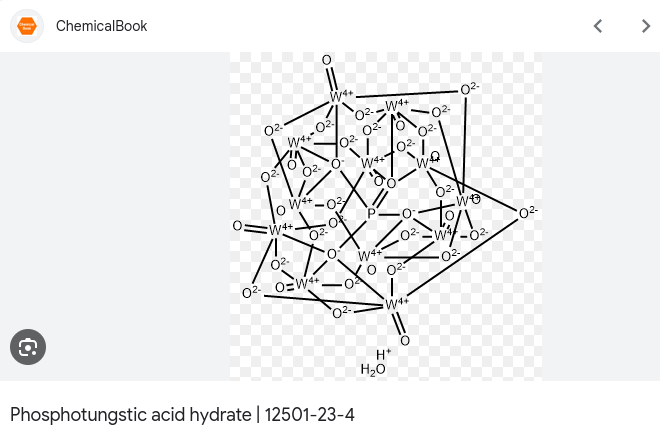
Tungstodicobaltate
In this article we are preparing a heteropolymetalate consisting of tungsten and cobalt, in particular the 11-Tungstodicobaltate (II), which we picked solely because “emerald green” sounded very pleasing to our ears.
Figuring out the structure of this particular compound was a tad annoying, because the 1956 paper by Baker et al4 actually got it wrong. The formula provided is (NH4)8[Co2W12O42] (a “12-tungstodicobaltate”), which implies a cobalt atom “slotted in” between the tungsten polyhedra on the edges (???). However, this is wrong, and a more modern reference5 indicates that the dicobaltate has the formula of (NH4)8[Co2(H2O)W11O39], adopting a lacunary Keggin structure with one of the edge tetrahedra being replaced with Cobalt instead of tungsten.
The preparation
The synthesis performed by us is based on the paper by Baker et al4, though we recommend looking at a more modern and optimized version of it by Barros et al.5
1.15 grams of cobalt acetate (0.0045 mol) were dissolved in 6 ml of distilled water and the solution was acidified with a few drops of glacial acetic acid. Another solution of 9.1 grams of sodium tungstate dihydrate (0.0276 mol) in 20 ml of water was also prepared, to which 2.1 ml of glacial acetic acid were added in order to get the pH to 6.5.
The tungstate solution was brought a boil and a warm solution of the cobalt acetate was slowly added, leading to a change in colour from clear to blue to deep emerald green. The resulting solution was boiled for 10 minutes and then gravity filtered while hot.
To the cooled down solution of the cobaltoditungstate was added a saturated solution of 6 grams of ammonium nitrate (0.075 mol), leading to the precipitation of our ammonium product. The solution was left to stand overnight in the fridge and a large amount of a shiny green precipitate was obtained. The precipitate was gravity filtered and washed with a little methanol, yielding 4.633 grams of dry crystalline tungstate, corresponding to a yield of 52%. (Another 0.480 g were obtained from the remaining mother liquor, giving us a total yield of 57%).

The last step in literature entailed 5 recrystallizations, which a dear friend of ours described as “advanced self harm”, and as much we dislike ourselves, the product obtained by a simple slow crystallization overnight contained no visible impurities, and therefore was kept as is without workup.
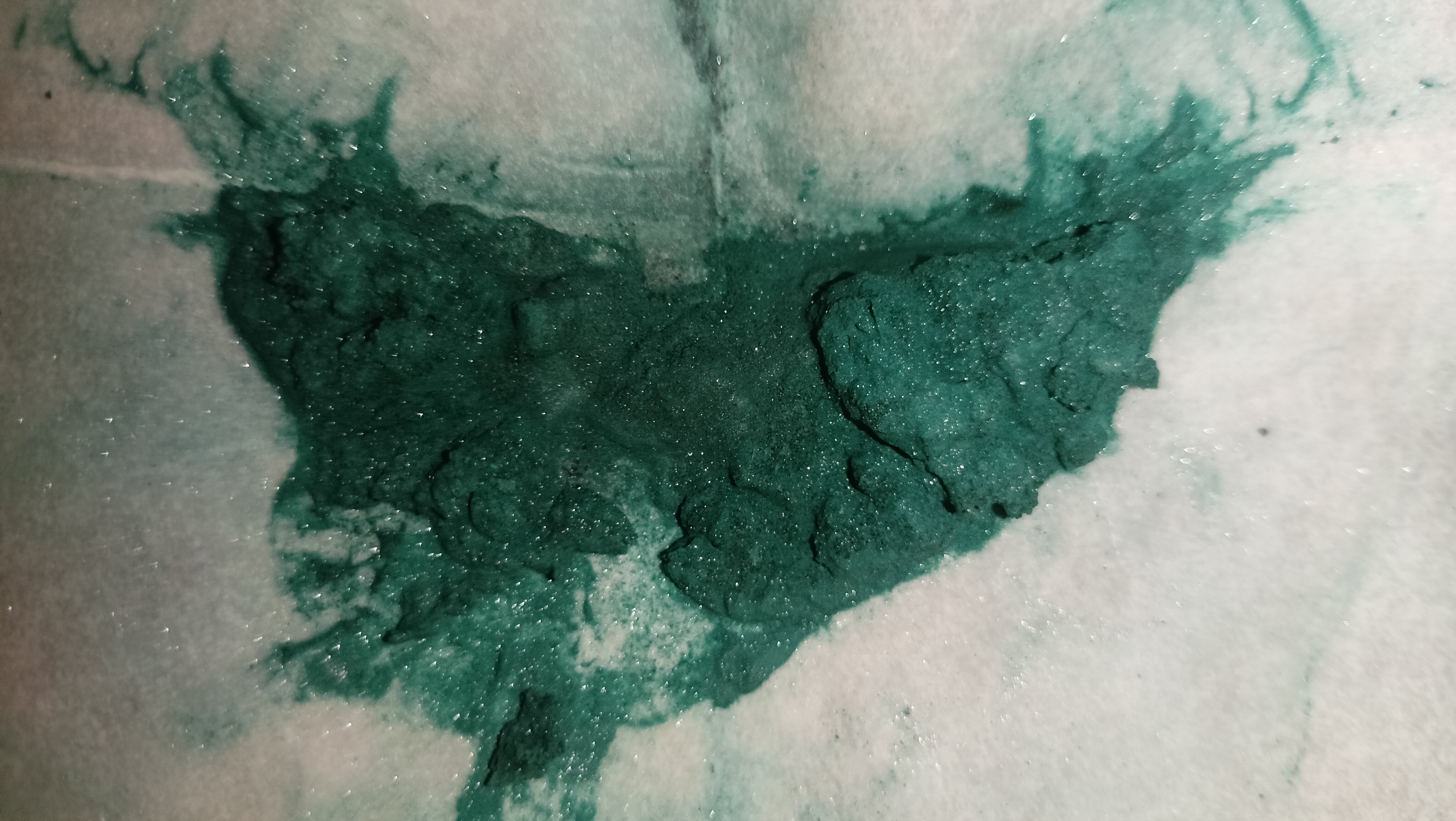
Taking a picture that actually displayed the sparkliness of the target compound was an exercise in utter misery, as the black cloth background we got off aliexpress.com isn’t well suited for photography in approaching-macro territory, and was promptly replaced with a sheet of sandpaper that spent the last 2 years inexplicably lying in a corner of our bedroom. (donations for camera equipment and such are very welcome)

What does it do?
Subjecting it to different harsh reaction conditions (strong acid, strong base), seems to mostly just ruin the polytungstate and turn it into tungsten trioxide or back into tungstate. A more detailed writeup of its chemistry is available in the cited prep and the redox/acid-base chemistry of this complex will be looked at in more detail in another post where we prepare some derivatives of this complex.
Sources
-
de Luyart, J.J. and F. (September 1783) “Análisis químico del volfram, y examen de un nuevo metal, que entra en su composición” (Chemical analysis of wolframite, and examination of a new metal, which enters into its composition), Extractos de las Juntas Generales celebradas por la Real Sociedad Bascongada de los Amigos del País en la ciudad de Vitoria por setiembre de 1783, pp. 46–88. https://archive.org/details/b21731032_0002 ↩
-
Ruiz Rubio, L., Vilas Vilela, J.L., Artetxe, B., & Gutiérrez-Zorrilla, J.M. (Eds.). (2022). Polyoxometalates: Advances, Properties, and Applications (1st ed.). Jenny Stanford Publishing. https://doi.org/10.1201/9781003277446 ↩
-
KEGGIN, J. F. (1933). Structure of the Molecule of 12-Phosphotungstic Acid. Nature, 131(3321), 908–909. https://doi.org/10.1038/131908b0 ↩
-
Baker, L. C. W. & McCutcheon, T. P. (1956) Heteropoly Salts Containing Cobalt and Hexavalent Tungsten in the Anion. J. Am. Chem. Soc. 78, 4503–4510. https://doi.org/10.1021/ja01599a001 ↩ ↩2
-
Barros Á, Artetxe B, Eletxigerra U, Aranzabe E, Gutiérrez-Zorrilla JM. Systematic Approach to the Synthesis of Cobalt-Containing Polyoxometalates for Their Application as Energy Storage Materials. Materials. 2023; 16(14):5054. https://doi.org/10.3390/ma16145054 ↩ ↩2